Main Research Interest: Chemical Toxicology and Biomarker Discovery
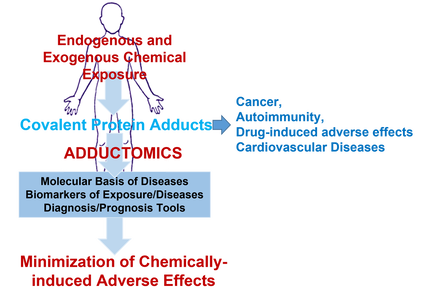
My work is focused on the Chemical Biology area with special emphasis on the domains of Chemical Toxicology and Biomarker Discovery.
Dedicated efforts have been placed on unrevealing the underlying molecular mechanisms of toxicity of chemical agents of endogenous, environmental, drug and dietary sources. This encompasses not only the identification of the metabolic fate of these agents but also the ability to covalently modifying DNA and proteins, forming covalent adducts. [AM1] This multidisciplinary work comprises the development of synthetic methodologies that mimic metabolic reactions, the synthesis and characterization of standards (metabolites and adducts formed with biomacromolecules), and the development of analytical methodologies capable of identifying and quantifying biomarkers of exposure to chemical agents.
This triggered the development of a unique set of skills, combining a basic background in organic chemistry, toxicology and analytical methodologies, with an emphasis on mass spectrometry and NMR techniques.
The motivation for this work is the translation of fundamental chemistry principles to address pivotal challenges in the clinical, forensic, and pharmacology areas. This objective is pursued through exploration of three distinct research lines:
- Molecular mechanisms underlying the toxicity of drugs (pharmacological and Psychoactive Active Substances) and environmental pollutants: from the metabolic fate to the formation of covalent adducts with proteins towards the identification of biomarkers of exposure/toxicity
- Development of new stratification/diagnosis tools for non-communicable diseases
- Medicinal Chemistry: development of anticancer organoselenium derivatives
MAIN RESEARCH TOPICS
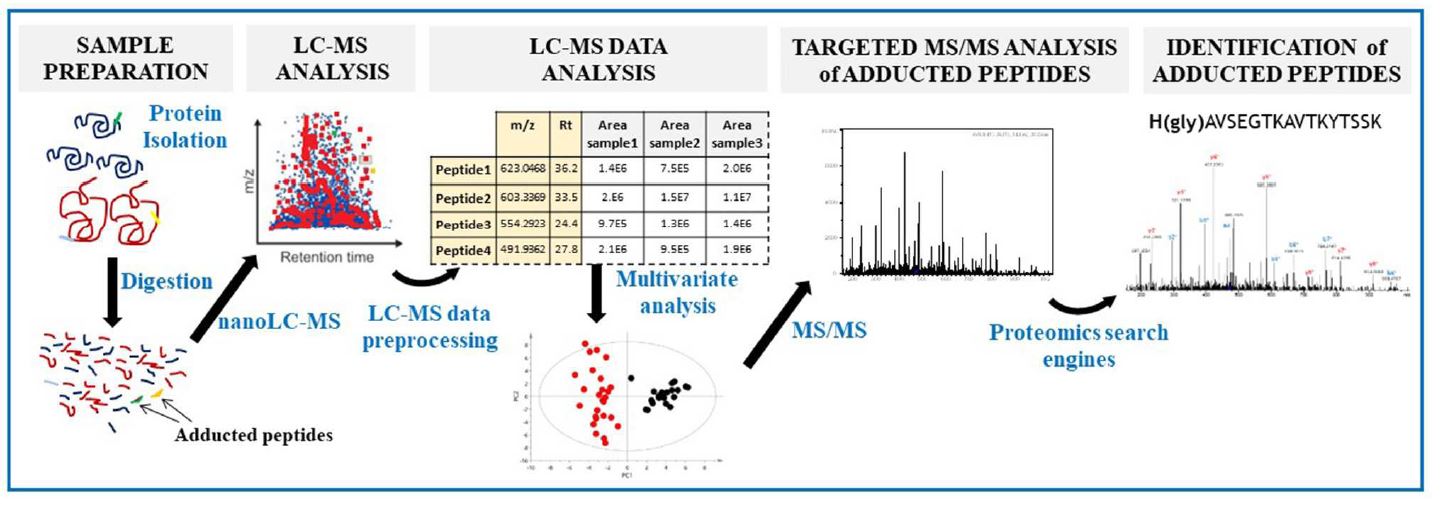
Mass spectrometry-based metodologies for the identification of protein covalent adducts
A Metabolomics-Inspired Strategy for the Identification of Protein Covalent Modifications
• Our novel strategy was able to differentiate HepG2 and THLE2 glycidamide- treated cells and to identify adducts that were not detected by the standard methodology of adductomics.
• This metabolomics driven approach in adductomics will not only open new opportunities for the identification of protein epigenetic modifications, but also adducts formed by endogenous and exogenous exposure to chemical agents.
Drug Metabolism and Bioactivation IN VIVO
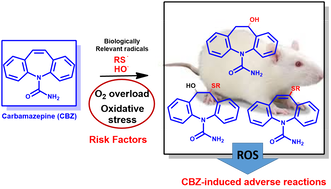
The first-line antiepileptic drug carbamazepine: Reaction with biologically relevant free radicals
•CBZ reacts with biologically relevant thiols with no need for bioactivation.
• The product profile is complex and consistent with radical-mediated mechanisms.
• The reaction is more extensive in the presence of oxygen.
• Products compatible with radical-mediated mechanisms were identified in liver extracts from CBZ-treated rats.
• The concomitant generation of reactive oxygen species can have a role on CBZ-induced adverse reactions.
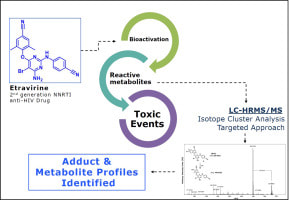
High resolution mass spectrometry-based methodologies for identification of Etravirine bioactivation to reactive metabolites: In vitro and in vivo approaches
|
|
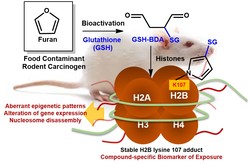
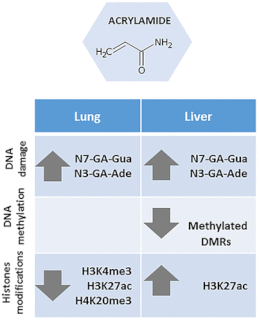
A furan-derived adduct was identified in liver histone 2B of furan-treated rats.
• This adduct was detected prior to the identification of epigenetic changes.
• This adduct represents an early compound-specific biomarker of exposure.
• The first evidence for in vivo occurrence of carcinogen-modified histones is provided.
• New insights into the mechanisms of chemical carcinogenesis are provided
Glycidamide and cis-2-butene-1,4-dial (BDA) as potential carcinogens and promoters of liver cancer - An in vitro study
Metabolite profilling and metabolic stability of New Psycoacthive Substances
Lopes RP, Ferro RA, Milhazes M, Figueira M, Caldeira MJ, Antunes AMM*, Gaspar H*, Metabolic stability and metabolite profiling of emerging synthetic cathinones. Front. Pharmacol. 2023, 14:1145140.
Lopes BT, Caldeira MJ, Gaspar H*, Antunes AMM* . Metabolic Profile of Four Selected Cathinones in Microrosome incubations: Identification of Phase I and II metabolites by Liquid Chromatography High Resolution Mass Spectrometry, Front Chem. 2021, 8:609251.
Biomonitoring (cells, animal models and humans) of drug-protein adducts by mass spectrometry-based tools
• Pinheiro PF, Pereira SA, Harjivan SG, Martins IL, Marinho AT, Cipriano M, Jacob CC, Oliveira NG, Castro MF, Marques MM, Antunes AMM*, Miranda JP*. Hepatocyte spheroids as a competent in vitro system for drug biotransformation studies: nevirapine as a bioactivation case study. Arch Toxicol. 2016, DOI :10.1007/s00204-016-1792-x.
•Grilo NM, Antunes AMM*, Caixas U, Marinho AT, Charneira C, Oliveira MC, Marques MM, Pereira SA*, Monitoring abacavir bioactivation in humans: screening for an aldehyde metabolite. Toxicol Lett 2013 219, 59-64.
• Caixas U, Antunes AMM*, Marinho AT, Godinho AL, Grilo NM, Marques MM, Oliveira MC, Branco T, Monteiro EC, Pereira SA*. Evidence for nevirapine bioactivation in man: searching for the first step in the mechanism of nevirapine toxicity. Toxicology 2012, 301, 33-39.
• Charneira C, Grilo NM, Pereira SA, Godinho ALA, Monteiro EC, Marques MM, Antunes AMM*. N-terminal valine adduct from the anti-HIV drug abacavir in rat hemoglobin as evidence for abacavir metabolism to a reactive aldehyde in vivo. British Journal of Pharmacology 2012, 167, 1353-1361.
• This adduct was detected prior to the identification of epigenetic changes.
• This adduct represents an early compound-specific biomarker of exposure.
• The first evidence for in vivo occurrence of carcinogen-modified histones is provided.
• New insights into the mechanisms of chemical carcinogenesis are provided
Glycidamide and cis-2-butene-1,4-dial (BDA) as potential carcinogens and promoters of liver cancer - An in vitro study
- HepG2 and THLE2 human liver cells were exposed to glycidamide and BDA.
- Surviving malignant cells exhibit growth advantage, suggesting cancer progression.
- Carcinogenic initiation was suggested by AFP overexpression in non-cancer cells.
- Glycidamide and BDA are putative cancer promoters.
Metabolite profilling and metabolic stability of New Psycoacthive Substances
Lopes RP, Ferro RA, Milhazes M, Figueira M, Caldeira MJ, Antunes AMM*, Gaspar H*, Metabolic stability and metabolite profiling of emerging synthetic cathinones. Front. Pharmacol. 2023, 14:1145140.
Lopes BT, Caldeira MJ, Gaspar H*, Antunes AMM* . Metabolic Profile of Four Selected Cathinones in Microrosome incubations: Identification of Phase I and II metabolites by Liquid Chromatography High Resolution Mass Spectrometry, Front Chem. 2021, 8:609251.
Biomonitoring (cells, animal models and humans) of drug-protein adducts by mass spectrometry-based tools
• Pinheiro PF, Pereira SA, Harjivan SG, Martins IL, Marinho AT, Cipriano M, Jacob CC, Oliveira NG, Castro MF, Marques MM, Antunes AMM*, Miranda JP*. Hepatocyte spheroids as a competent in vitro system for drug biotransformation studies: nevirapine as a bioactivation case study. Arch Toxicol. 2016, DOI :10.1007/s00204-016-1792-x.
•Grilo NM, Antunes AMM*, Caixas U, Marinho AT, Charneira C, Oliveira MC, Marques MM, Pereira SA*, Monitoring abacavir bioactivation in humans: screening for an aldehyde metabolite. Toxicol Lett 2013 219, 59-64.
• Caixas U, Antunes AMM*, Marinho AT, Godinho AL, Grilo NM, Marques MM, Oliveira MC, Branco T, Monteiro EC, Pereira SA*. Evidence for nevirapine bioactivation in man: searching for the first step in the mechanism of nevirapine toxicity. Toxicology 2012, 301, 33-39.
• Charneira C, Grilo NM, Pereira SA, Godinho ALA, Monteiro EC, Marques MM, Antunes AMM*. N-terminal valine adduct from the anti-HIV drug abacavir in rat hemoglobin as evidence for abacavir metabolism to a reactive aldehyde in vivo. British Journal of Pharmacology 2012, 167, 1353-1361.
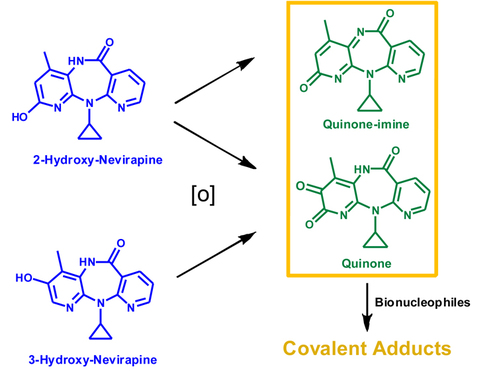
Synthesis and Structural Characterization of drug metabolites, DNA and protein adduct standards
Selected Related Publications:
We investigated the ability of two phenolic NVP metabolites,
2-hydroxy-NVP and 3-hydroxy-NVP, to undergo oxidation and subsequent reaction with bionucleophiles: 2-hydroxy-NVP yielded a quinone-imine and a quinone and 3-hydroxy-NVP yielded only the quinone. Both quinoide derivatives reacted with nitrogen-based bionucleophiles.
The metabolic conversion of phenolic NVP metabolites into quinoid electrophiles is biologically plausible.: as the use of oxidases (lactoperoxidase, myeloperoxidase and tyrosinase) yielded product profiles similar to the ones observed upon Frémy’s salt oxidation
The lack of reaction with sulfhydryl groups might hamper the in vivo detoxification of NVP-derived quinone and quinone-imine metabolites via glutathione conjugation.
These metabolites could be available for reaction with nitrogen-based bionucleophiles(e.g., lysine residues of proteins) ultimately eliciting toxic events
Harjivan SH, Pinheiro PF, Martins IL, Godinho AL, Wanke R, Santos PP, Pereira SA, Beland FA, Marques MM, Antunes AMM*, Quinoid derivatives of the nevirapine metabolites 2-hydroxy- and 3-hydroxy-nevirapine: activation pathway to amino acid adduct. Toxicology Research, 2015, 4, 1565 - 1577.
Harjivan SH, Wanke R; Ferreira da Silva JL; Marques MM, Antunes AMM*, The phenolic metabolites of the anti-HIV drug efavirenz: evidence for distinct reactivities upon oxidation with Frémy's salt. Eur J Med Chem, 2014, 74, 7–11.
Charneira C, Godinho ALA, Oliveira MC, Pereira SA, Monteiro EC, Marques MM, Antunes AMM*, Reactive Aldehyde Metabolites from the Anti-HIV Drug Abacavir: Amino Acid Adducts as Possible Factors in Abacavir Toxicity, Chemical Research in Toxicology, 2011, 24, 2129-2141.
Antunes AMM*, Godinho ALA, Martins IL, Oliveira MC, Gomes RA, Coelho AV, Beland FA, Marques MM, Protein adducts as prospective biomarkers of nevirapine toxicity, Chemical Research in Toxicology, 2010, 23 ,1714–1725.
Antunes AMM*, Duarte MP, Santos PP, Gamboa da Costa G, Heinz TM, Beland FA, Marques MM, Synthesis and Characterization of DNA Adducts from the HIV Reverse Transcriptase Inhibitor Nevirapine, Chemical Research in Toxicology, 2008, 21, 1443–1456.
Selected Related Publications:
We investigated the ability of two phenolic NVP metabolites,
2-hydroxy-NVP and 3-hydroxy-NVP, to undergo oxidation and subsequent reaction with bionucleophiles: 2-hydroxy-NVP yielded a quinone-imine and a quinone and 3-hydroxy-NVP yielded only the quinone. Both quinoide derivatives reacted with nitrogen-based bionucleophiles.
The metabolic conversion of phenolic NVP metabolites into quinoid electrophiles is biologically plausible.: as the use of oxidases (lactoperoxidase, myeloperoxidase and tyrosinase) yielded product profiles similar to the ones observed upon Frémy’s salt oxidation
The lack of reaction with sulfhydryl groups might hamper the in vivo detoxification of NVP-derived quinone and quinone-imine metabolites via glutathione conjugation.
These metabolites could be available for reaction with nitrogen-based bionucleophiles(e.g., lysine residues of proteins) ultimately eliciting toxic events
Harjivan SH, Pinheiro PF, Martins IL, Godinho AL, Wanke R, Santos PP, Pereira SA, Beland FA, Marques MM, Antunes AMM*, Quinoid derivatives of the nevirapine metabolites 2-hydroxy- and 3-hydroxy-nevirapine: activation pathway to amino acid adduct. Toxicology Research, 2015, 4, 1565 - 1577.
Harjivan SH, Wanke R; Ferreira da Silva JL; Marques MM, Antunes AMM*, The phenolic metabolites of the anti-HIV drug efavirenz: evidence for distinct reactivities upon oxidation with Frémy's salt. Eur J Med Chem, 2014, 74, 7–11.
Charneira C, Godinho ALA, Oliveira MC, Pereira SA, Monteiro EC, Marques MM, Antunes AMM*, Reactive Aldehyde Metabolites from the Anti-HIV Drug Abacavir: Amino Acid Adducts as Possible Factors in Abacavir Toxicity, Chemical Research in Toxicology, 2011, 24, 2129-2141.
Antunes AMM*, Godinho ALA, Martins IL, Oliveira MC, Gomes RA, Coelho AV, Beland FA, Marques MM, Protein adducts as prospective biomarkers of nevirapine toxicity, Chemical Research in Toxicology, 2010, 23 ,1714–1725.
Antunes AMM*, Duarte MP, Santos PP, Gamboa da Costa G, Heinz TM, Beland FA, Marques MM, Synthesis and Characterization of DNA Adducts from the HIV Reverse Transcriptase Inhibitor Nevirapine, Chemical Research in Toxicology, 2008, 21, 1443–1456.
Application of NMR tools to asses drug-protein interactions
Selected Related Publications:
Wanke R*, Harjivan SG, Pereira SA, Marques MM, Antunes AMM*, The role of competitive binding to human serum albumin on efavirenz-warfarin interaction: a nuclear magnetic resonance study, International Journal of Antimicrobial Agents 2013, 42, 443-444.
Wanke R*, Harjivan SG, Pereira SA, Marques MM, Antunes AMM*, The role of competitive binding to human serum albumin on efavirenz-warfarin interaction: a nuclear magnetic resonance study, International Journal of Antimicrobial Agents 2013, 42, 443-444.
Development of new synthetic approaches for the preparation of drug metabolites namely using bio-inspired catalysts.
Selected Related Publications:
Wanke R, Novais DA, Harjivan SG, Marques MM, Antunes AMM*, Biomimetic oxidation of aromatic xenobiotics: synthesis of the phenolic metabolites from the anti-HIV drug Efavirenz, Organic & Biomolecular Chemistry, 2012, 10, 4554-4561. IF: 3.5 [2Q1; 11; Chemistry, Organic]. Classified as “Hot Paper” and highlighted at OBC blog
Selected Related Publications:
Wanke R, Novais DA, Harjivan SG, Marques MM, Antunes AMM*, Biomimetic oxidation of aromatic xenobiotics: synthesis of the phenolic metabolites from the anti-HIV drug Efavirenz, Organic & Biomolecular Chemistry, 2012, 10, 4554-4561. IF: 3.5 [2Q1; 11; Chemistry, Organic]. Classified as “Hot Paper” and highlighted at OBC blog
Synthetic/Medicinal Chemistry
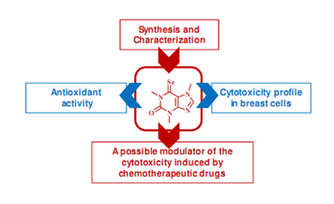
Selected Related Publications:
Martins IL, Charneira C, Gandin V, Ferreira da Silva JL , Justino GC, Telo JP, Vieira AJSC, Marzano C, Antunes AMM*, Selenium-containing Chrysin and Quercetin Derivatives: Attractive scaffolds for cancer therapy. J Med Chem, 2015, 58, 4250-4265..
dos Santos EA, Hamel E, Bai R, Burnett J, Santos CC, Tozatti S, Bogo D, Perdomo RT, Antunes AMM, Marques MM, Matos MFC, de Lima DP*, Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorganic & Medicinal Chemistry Letters, 2013 15. 23, 4669-4673.
Martins IL, Miranda JP, Oliveira NG, Fernandes AS, Gonçalves S, Antunes AMM*, Synthesis and biological activity of 6-selenocaffeine: potential modulator of chemotherapeutic drugs in breast cancer cells. Molecules 2013, 18, 5251-5264.
Martins IL, Charneira C, Gandin V, Ferreira da Silva JL , Justino GC, Telo JP, Vieira AJSC, Marzano C, Antunes AMM*, Selenium-containing Chrysin and Quercetin Derivatives: Attractive scaffolds for cancer therapy. J Med Chem, 2015, 58, 4250-4265..
dos Santos EA, Hamel E, Bai R, Burnett J, Santos CC, Tozatti S, Bogo D, Perdomo RT, Antunes AMM, Marques MM, Matos MFC, de Lima DP*, Synthesis and evaluation of diaryl sulfides and diaryl selenide compounds for antitubulin and cytotoxic activity. Bioorganic & Medicinal Chemistry Letters, 2013 15. 23, 4669-4673.
Martins IL, Miranda JP, Oliveira NG, Fernandes AS, Gonçalves S, Antunes AMM*, Synthesis and biological activity of 6-selenocaffeine: potential modulator of chemotherapeutic drugs in breast cancer cells. Molecules 2013, 18, 5251-5264.